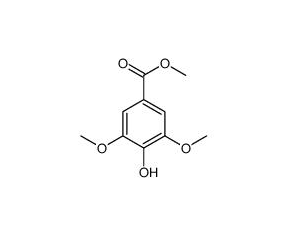
Methyl syringate
CAS No. 884-35-5
Methyl syringate( —— )
Catalog No. M19228 CAS No. 884-35-5
Methyl syringate has a unique inhibitory activity toward aflatoxin production with a different mode of action from that of gallic acid.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | 35 | In Stock |


|
| 500MG | 82 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameMethyl syringate
-
NoteResearch use only, not for human use.
-
Brief DescriptionMethyl syringate has a unique inhibitory activity toward aflatoxin production with a different mode of action from that of gallic acid.
-
DescriptionMethyl syringate has a unique inhibitory activity toward aflatoxin production with a different mode of action from that of gallic acid. Methyl syringate from K. pictus is a specific and selective activator of hTRPA1, can regulate food intake and gastric emptying through a TRPA1-mediated pathway and, by extension, can contribute to weight suppression.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorhTRPA1
-
Research AreaOthers-Field
-
Indication——
Chemical Information
-
CAS Number884-35-5
-
Formula Weight212.2
-
Molecular FormulaC10H12O5
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (471.25 mM)
-
SMILESCOc1cc(cc(c1O)OC)C(=O)OC
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Kim MJ, et al. PLoS One. 2013 Aug 21;8(8):e71603.
molnova catalog



related products
-
Calcium pantothenate
D-Pantothenic acid hemicalcium salt, a kind of water soluble vitamin, can reduce the patulin content of the apple juice.
-
Capryl caprate
Capryl caprate (Decanoic acid decyl ester) is a compound obtained from extracts of Tergite glands from the abdomen of virgin queen bees.
-
p-Hydroxy-5,6-dehydr...
p-Hydroxy-5,6-dehydrokawain is isolated from Kawain.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


